Tight spaces cause spreading cancer cells to divide improperly
Scientists have found a new way to study how cancer cells divide and thrive in difficult-to-reach crannies of the body.
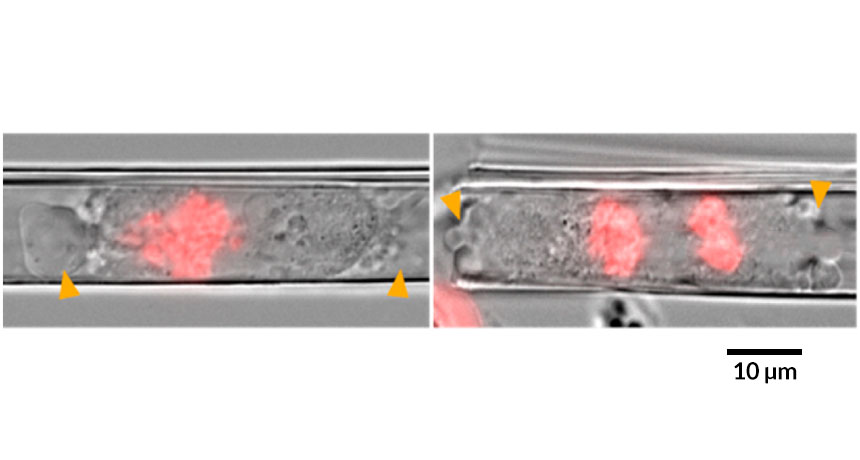
Transparent artificial membranes — just nanometers thick — can be rolled into tubes to mimic capillaries that host spreading cancer cells, researchers report in the June ACS Nano. Cells squished inside such tubes didn’t organize their internal components the way they normally do before splitting. As a result, the cells divided unevenly, potentially introducing new mutations.
Inside the body, cancer cells fight for space. Sometimes they’ll spread, or metastasize, to other organs via tight blood vessels. Although cancer cells are more likely to kill once they spread, scientists still don’t understand how the abnormal cells divide inside such tiny tubes. These cells are difficult to study in the body because they’re tucked away in hard-to-reach places. They’re challenging to study in the lab, too, because they behave differently in a petri dish than in their natural environment.
By replicating that environment more closely, this experiment gives “an appreciation for what it’s like to be a cell in a body,” says Buzz Baum, a biologist at University College London who was not involved in the work.
Other researchers have looked at cells constrained in other ways. But the nanotubes are round, just like blood vessels. They’re transparent, making it easier to visualize what’s happening inside. And it’s possible to study many cells at once by growing nearly a thousand tubes, all exactly the same size, on a chip slightly larger than a postage stamp.
When watching single human cancer cells inside the tubes through high-powered microscopes, the team noticed that the squished cells didn’t divide symmetrically. Instead of sending half the chromosomes to each new cell, some of those cells got extra genetic instructions, while others were shortchanged.
The squished cells also took longer to divide. And the protein structures that help guide the chromosomes and organelles into place before division didn’t develop correctly, says study coauthor Wang Xi, a materials scientist who did the work at the Leibniz Institute for Solid State and Materials Research Dresden in Germany.
Cancer cells succeed by mutating enough that they can evade capture, without becoming too mutated to keep replicating.
“A cell that makes too many mistakes will just die,” says Baum.
When cells are trapped inside blood vessels, “they become misshapen but they’re still able to divide,” says Xi, now at the National University of Singapore.
Xi and his colleagues think that a bulging of the cell membrane in response to pressure, called blebbing, might help the trapped cells divide in a slightly less distorted way. When the researchers prevented the cells from blebbing, the division was even more uneven. But because the team can’t yet explain why this would be the case, it’s too soon to say whether blebbing itself is responsible for the improved division.
Baum says he has shown similar deformities in cancer cells dividing under other types of constraints. But, he adds, it’s important to have systems that more closely replicate the body’s internal environment. Otherwise, it can be a big jump between doing tests in a petri dish and in a live animal.
Study coauthor Christine Schmidt, a biochemist at the University of Cambridge, says understanding how cancer cells manage to divide in tight quarters could eventually inspire ways to kill spreading cancer cells without hurting healthy cells.